Education:
Ph.D.: 2010, Microbiology and Immunology, University of Michigan-Ann Arbor
Pre-OUHSC:
Department of Microbiology, University of Washington - Seattle
Research Interests:
Bacterial pathogenesis, innate immunity, antimicrobial resistance, membrane lipid remodeling, biochemistry of transmembrane proteins, bacterial glycerophospholipids.
Contact Information:
Office: BMSB 920
Email: Zachary-Dalebroux@ouhsc.edu
Research Interests:
The cell envelope of Gram-negative bacteria comprises an inner and outer membrane (OM) separated by a periplasmic space containing a thin layer of peptidoglycan, which is bound to the OM by lipoproteins. The OM itself is an asymmetric lipid bilayer of inner-leaflet glycerophospholipids and outer-leaflet lipid A molecules interrupted by β-barrel proteins. Fatty-acyl chains of glycerophospholipids and lipid A engage in hydrophobic interactions that anchor long negatively charged polar lipopolysaccharides (LPS), and capsule-forming exopolysaccharides to the microbial cell surface. The OM acts as a barrier to the environment that allows the bacterium to control the passage of ions, solutes, metabolites, macromolecules, and antibiotics into and out of the cell. Mechanisms for the export and assembly of OM proteins, LPS, and capsule have been characterized. By contrast, how bacteria transport, assemble, and remodel OM-glycerophospholipids for barrier function and cell growth has remained a mystery. In general, Dr. Dalebroux’s research laboratory will interrogate (i) how OM-glycerophospholipids are regulated for bacterial survival and resistance to stress, (ii) the mechanisms of membrane lipid trafficking and remodeling necessary for bacterial growth and survival during infection and (iii) how OM-glycerophospholipids interact with the protein, lipid A, and polysaccharide components of the OM to promote bacterial resistance to antibiotics and the innate immune system.
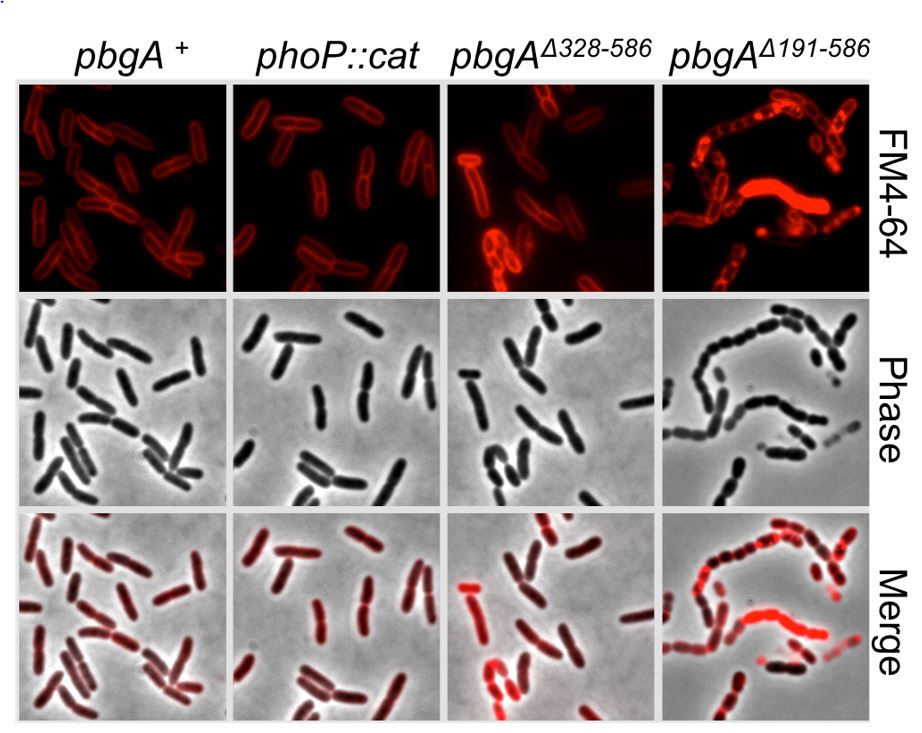
Salmonella enterica serovar Typhimurium is a Gram-negative bacterial pathogen that causes foodborne gastroenteritis in healthy humans and more severe systemic disease in immunocompromised hosts. As part of their pathogenesis, S. Typhimurium rely upon regulation of OM glycerophospholipid content to survive within the acidified vacuoles of macrophages during systemic virulence for mice. Genetic screening has identified several interesting S. Typhimurium proteins involved in OM-glycerophospholipid remodeling for barrier function, many of unknown function. These include a conserved enterobacterial gene product encoding a transmembrane protein known as, PhoPQ barrier gene A (PbgA). PbgA contains a periplasmic domain that binds acidic glycerophospholipids known as cardiolipins near the bacterial inner membrane and promotes their trafficking to the OM on activation of the PhoPQ-virulence system. The PhoPQ regulators sense cationic antimicrobial peptides and acidic pH within macrophage phagolysosomes to activate the OM remodeling process. S. Typhimurium require regulated delivery of cardiolipins to the OM to survive in macrophages and to colonize the spleens of mice during systemic virulence. Additionally, the five transmembrane segments of PbgA contribute an undefined biochemical function that is essential for enterobacterial cell growth and division.
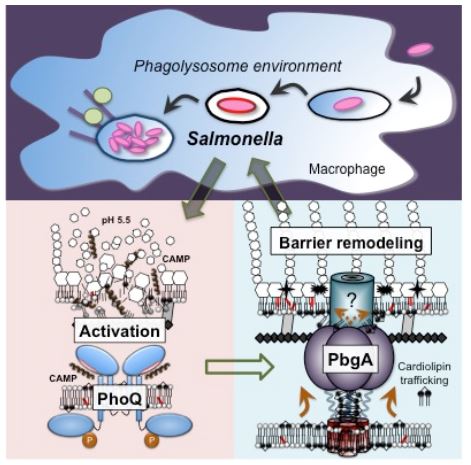
Presently, two avenues of research are being pursued in parallel. First, what is the biochemical function related to OM-glycerophospholipid remodeling and role in pathogenesis for the other PhoPQ barrier genes (Pbg) that were identified in the original genetic screen? What is the biochemical mechanism by which the PbgA transmembrane segments are essential for enterobacterial cell growth and division? In investigating OM-glycerophospholipid remodeling for Gram-negative bacterial pathogenesis, Dr. Dalebroux’s laboratory aims to fill a major gap in our current understanding of bacterial membrane physiology, antimicrobial resistance, and the host-pathogen dynamic in general.
Current Lab Personnel:
Melina Cian, Ph.D., Postdoctoral Research Fellow
Nicole Giordano, Graduate Student
Selected Publications:
- Masilamani, R, Cian, MB, Dalebroux, Z.D. Salmonella Tol-Pal Reduces Outer Membrane Glycerophospholipid Levels for Envelope Homeostasis and and Survival during Bacteremia. Infect Immun. 2018 Jul; 86(7)PubMed PMID: 29735519; PubMed Central PMCID: PMC6013679
- Dalebroux, Z.D. Cues from the Membrane: Bacterial Glycerophospholipids. J Bacteriol. 2017 Jul 1; 199(13)PubMed PMID: 28439041; PubMed Central PMCID: PMCID: PMC5472817.
- *Dalebroux, Z. D., Edrozo, M. B., Pfuetzner, R. A. Ressl, S., Kulasekara, B. R., Blanc, M. P., and S. I. Miller, Delivery of cardiolipins to the Salmonella outer membrane is necessary for survival in host tissues and virulence. 2015. Cell Host Microbe, 8;17(4):441-51.
- *Dalebroux, Z. D., and Miller S. I. Salmonellae PhoPQ regulation of the outer membrane to resist innate immunity. 2014. Curr. Opin. Microbiol. 17C:106-113.
- *Dalebroux, Z. D., Matamouros, S., Whittington, D., Bishop, R. E., and S. I. Miller. 2014. PhoPQ regulates acidic glycerophospholipid content of the Salmonella Typhimurium outer membrane. Proc. Natl. Acad. Sci. U S A. 5:1963-1968.
- Dalebroux, Z. D., and Swanson, M. S. 2012. ppGpp: Magic beyond RNA polymerase. Nat. Rev. Microbiol. 10:203-12.
- Dalebroux, Z. D., Svensson, S. L., Gaynor, E. C., and M. S. Swanson. 2010. ppGpp conjures bacterial virulence. Microbiol. Mol. Biol. Rev. 74: 171-99.
- Dalebroux, Z. D., Yagi, B. F., Sahr, T., Buchreiser, C., and M. S. Swanson. 2010. Distinct roles of ppGpp and DksA in Legionella pneumophila differentiation. Mol. Microbiol. 76: 200-219.
- Edwards, R. L., Dalebroux, Z. D., and M. S. Swanson. 2009. Legionella pneumophila couples fatty acid flux to microbial differentiation and virulence. Mol. Microbiol. 71: 1190-1204.
- Dalebroux, Z. D., Edwards, R. L., and M. S. Swanson. 2009. SpoT governs Legionella pneumophila differentiation in host macrophages. Mol. Microbiol. 71: 640-658.
- Suzuki, M. R., Hunt, C. G., Houtman, C. J., Dalebroux, Z. D., and K. E. Hammel. 2006. Fungal hydroquinones contribute to wood decay. Environ. Microbiol. 8: 2214-2223.